Key Points
- The human immunodeficiency virus (HIV) is the virus that causes HIV infection. If untreated, HIV may progress to AIDS, the most advanced stage of HIV infection.
- HIV medicines (called antiretroviral therapy, or ART) are typically a combination of medicines taken every day (as one or more pills) or every two weeks to six months (as injections).
- ART reduces the amount of HIV in the blood but does not cure HIV infection. ART is recommended for everyone with HIV to help support a long, healthy life.
- When taken consistently, ART can reduce the chances of HIV transmission through sex to zero and less than 1 percent while breastfeeding; untreated HIV is more likely to be spread through sex, needle sharing, pregnancy, and breastfeeding.
What is HIV and AIDS?
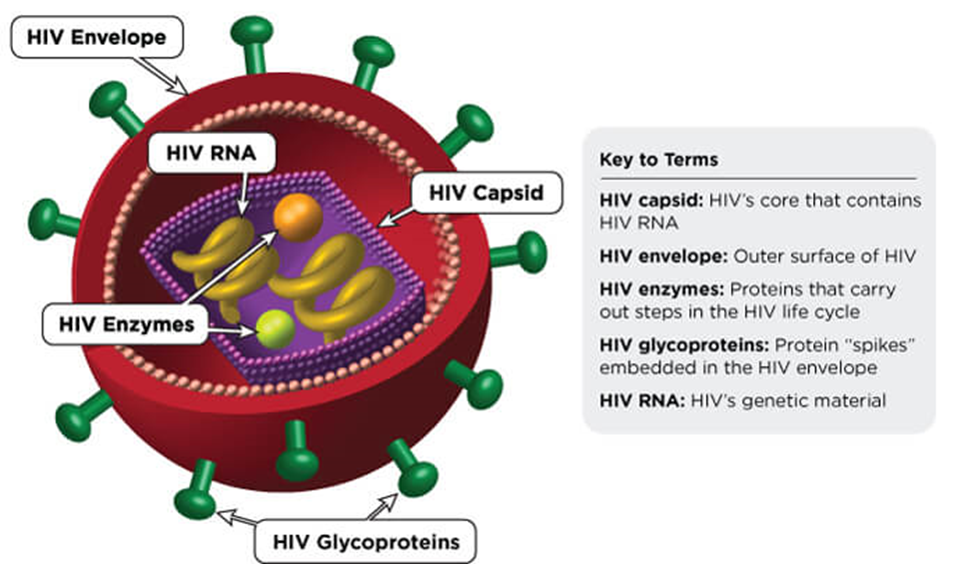
HIV stands for human immunodeficiency virus, which is the virus that causes HIV infection. The acronym “HIV” can refer to the virus or to HIV infection.
AIDS stands for acquired immunodeficiency syndrome. AIDS is the most advanced stage of HIV infection.
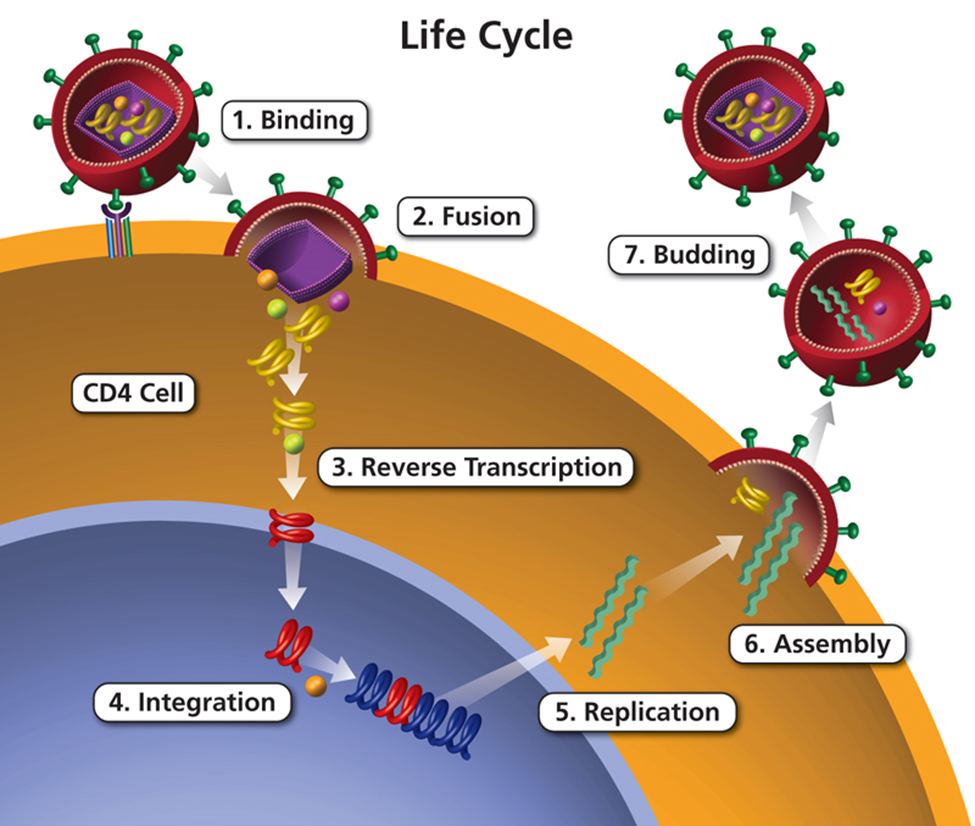
HIV attacks and destroys the infection-fighting CD4 cells (CD4 T lymphocytes) of the immune system. The loss of CD4 cells makes it difficult for the body to fight off infections, illnesses, and certain cancers.
Without treatment, HIV can gradually destroy the immune system, and may cause a decline in health or progress to AIDS. This includes an increased likelihood of getting other infections like hepatitis, tuberculosis, and some sexually transmitted infections.
With treatment, the body can prevent HIV from destroying CD4 cells, allowing the immune system to recover and protect against other infections.
See the HIVinfo The Stages of HIV Infection fact sheet for more information on how HIV progresses to AIDS.
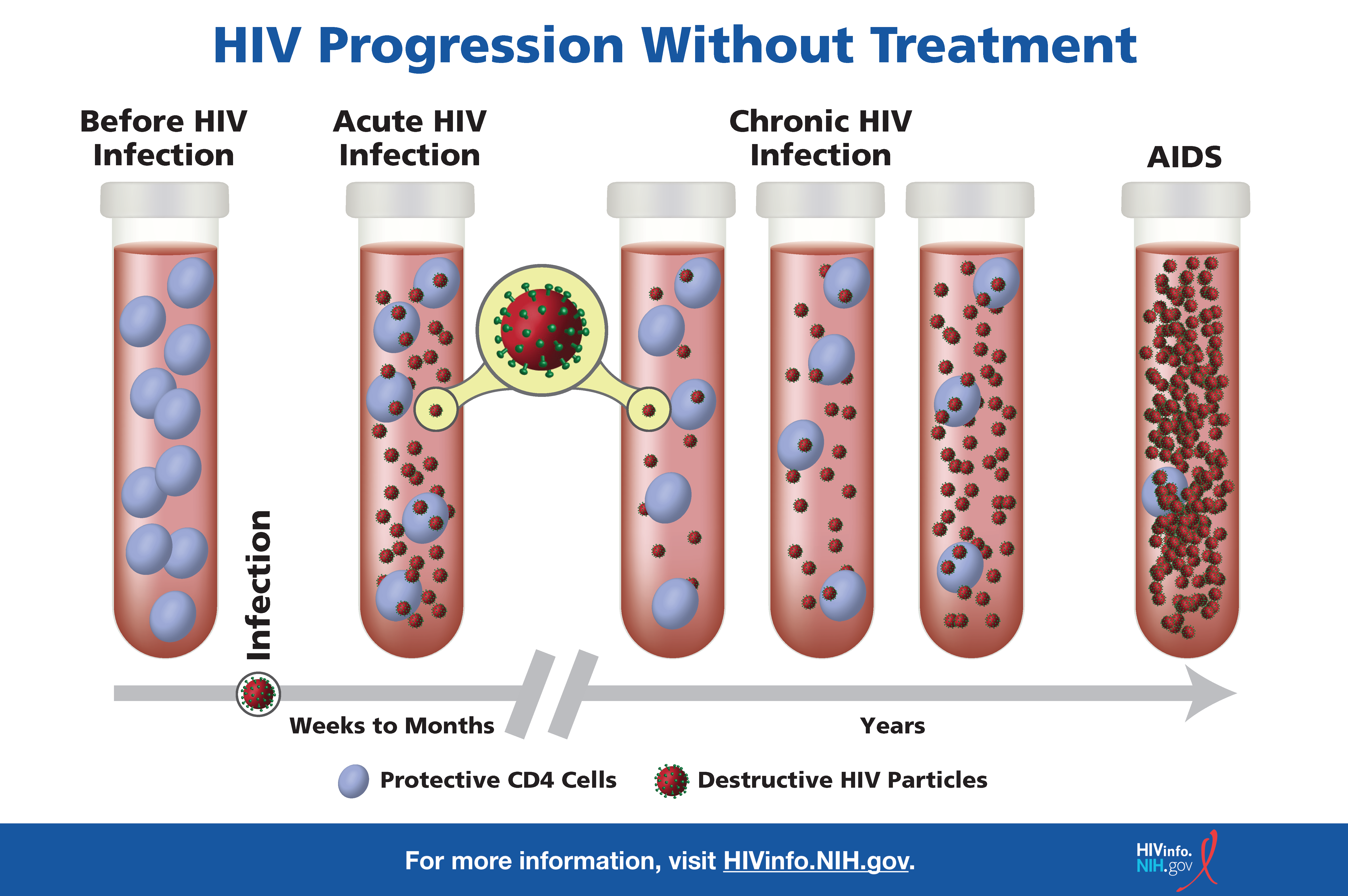
![The image depicts what happens in the blood of people with HIV throughout the stages of HIV infection.]()
How is HIV transmitted?
HIV can be transmitted through bodily fluids, including blood, semen (“cum”), pre-seminal fluid (“precum”), vaginal fluids, rectal fluids, and breastmilk. HIV can be transmitted through these fluids in the following ways—
Blood:
- Sharing needles or syringes for injecting drugs or tattooing
- Being exposed to the blood of a person with HIV through accidental needle sticks
- Sharing personal items that may have blood on them (such as razors or toothbrushes)
- Eating prechewed foods (in case of open sores or bleeding gums)
Semen, Preseminal Fluid, Vaginal Fluids, and Rectal Fluids:
- Having vaginal or anal sex
- Having oral sex (less likely, but can occur in rare cases)
When an infant gets HIV during pregnancy, childbirth, or breastfeeding, it is called perinatal transmission of HIV. For more information on perinatal transmission, read the HIVinfo Preventing Perinatal Transmission of HIV fact sheet.
You cannot get HIV by shaking hands or hugging a person who has HIV. You also cannot get HIV from contact with objects, such as dishes, toilet seats, or doorknobs, used by a person with HIV. HIV is not spread through the air or water or by mosquitoes, ticks, or other insects.
Use the HIVinfo You Can Safely Share…With Someone With HIV infographic to spread this message.
What is the treatment for HIV?
Antiretroviral therapy (ART) is the use of HIV medicines to treat HIV. People on ART take a combination of HIV medicines (called an HIV treatment regimen) every day (pills) or on a schedule (injections).
In many cases, oral medicines may be combined into a single pill or capsule instead of multiple pills. There are newer long-acting medicines given by injection every two weeks to six months that may be appropriate for some people with HIV.
Although injectable HIV medicines are taken less often, they require an appointment to be administered by a health care provider. See the Long-Acting HIV Medicines fact sheet for more information.
ART is recommended for everyone who has HIV to prevent the virus from multiplying, which reduces the amount of HIV in the body (called the viral load). Having less HIV in the body protects the immune system and prevents HIV from advancing to AIDS. ART cannot cure HIV, but it can help people with HIV live long, healthy lives.
How can a person reduce the chances of transmitting HIV?
ART reduces the chances of HIV transmission. ART can reduce a person’s viral load to an undetectable level. An undetectable viral load means that the level of HIV in the blood is too low to be detected by a viral load test.
People with HIV who maintain an undetectable viral load have no risk of transmitting HIV to their partners through sex. This is known as Undetectable = Untransmittable, or U=U.
HIV medicines taken during pregnancy, childbirth, and breastfeeding can also reduce the likelihood of perinatal HIV transmission. Evidence suggests that the odds of perinatal transmission are less than 1 percent when breastfeeding with an undetectable viral load.
Feeding infants using properly prepared formula or pasteurized human donor milk from a milk bank can eliminate the chances of perinatal HIV transmission completely. Health care providers can help determine the best approach to infant feeding based on the likelihood that an infant will be exposed to HIV after pregnancy.
How can a person reduce the risk of getting HIV?
There are several ways to reduce the risk of getting HIV:
- Using condoms correctly with every sexual encounter, particularly with partners who have HIV with a detectable viral load or whose HIV status is unknown
- Avoiding needle sharing
- Choosing safer sex practices (such as limiting the number of sexual partners)
People who do not have HIV should talk to their health care provider about pre-exposure prophylaxis (PrEP) if they believe they are at risk of getting HIV. PrEP involves taking a specific HIV medicine on a routine schedule—daily for pills or by schedule for injections—before an HIV exposure. For more information, read the HIVinfo Pre-Exposure Prophylaxis (PrEP) fact sheet.
Post-exposure prophylaxis (PEP) can also reduce the chances of getting HIV. Unlike PrEP, PEP is taken within three days (72 hours) after an exposure. Read the HIVinfo Post-Exposure Prophylaxis (PEP) fact sheet for more information.
What are the symptoms of HIV and AIDS?
Within two to four weeks after infection with HIV (known as early or acute HIV infection), some people may have flu-like symptoms (such as fever or chills). The symptoms may last for a few days to several weeks.
Other possible symptoms of HIV include night sweats, muscle aches, sore throat, fatigue, swollen lymph nodes, headache, diarrhea, mouth ulcers, and skin rash. Having these symptoms does not mean you have HIV. Other illnesses can cause the same symptoms.
Some people may not feel sick during early HIV infection. During this earliest stage of HIV infection, the virus multiplies rapidly. After the initial stage of infection, HIV continues to multiply but at lower levels.
When people with HIV do not receive treatment, more severe HIV symptoms may appear years later, as HIV develops into AIDS. People with AIDS have weakened immune systems that make them prone to opportunistic infections.
Opportunistic infections are infections and infection-related cancers that occur more frequently or are more severe in people with weakened immune systems than in people with healthy immune systems. See the What is an Opportunistic Infection? fact sheet to learn more.
Without treatment, HIV transmission is possible at any stage of HIV infection—even if a person with HIV has no symptoms.
How is AIDS diagnosed?
Symptoms such as fever, weakness, and weight loss may be a sign that a person’s HIV has advanced to AIDS. However, an AIDS diagnosis is based on the following criteria:
Although an AIDS diagnosis indicates severe damage to the immune system, HIV medicines can still help people at this stage of HIV infection.
This fact sheet is based on information from the following sources:
From the Centers for Disease Control and Prevention:
From the HIV Clinical Practice Guidelines at Clinicalinfo.HIV.gov:
- Guidelines for the Use of Antiretroviral Agents in Pediatric HIV Infection:
- Recommendations for the Use of Antiretroviral Drugs During Pregnancy and Interventions to Reduce Perinatal HIV Transmission in the United States
From MedlinePlus:
From the National Institute of Allergy and Infectious Diseases (NIAID):
Also see the HIV Source collection of HIV links and resources.