What is an Investigational HIV Drug?
Key Points
- An investigational drug is a drug that is being studied to determine whether it is safe and effective and how much of the drug is needed to treat a disease or medical condition, such as HIV.
- Investigational HIV drugs are studied in a series of medical studies known as clinical trials. Once an investigational HIV drug has been proven safe and effective, the U.S. Food and Drug Administration may approve the drug for general use or sale.
- Investigational HIV drugs include both drugs and vaccines used to prevent or treat HIV. These drugs can only be accessed through clinical trials and expanded access programs.
What is an investigational HIV drug?
As defined by the U.S. Food and Drug Administration (FDA), an investigational HIV drug is an experimental drug that is being studied to determine:
- Whether the drug is safe and effective
- If and how the drug may be used in a specific population (such as adults, children, pregnant women)
- How much of the drug is needed
- Potential benefits and risks of taking the drug
Investigational HIV drugs are studied in a series of medical research studies called clinical trials to determine if they are safe, if they work, and how they should be used. Clinical trials progress through three phases before drug sponsors submit a New Drug Application to the FDA for formal review.
If the FDA determines that the drug meets regulatory standards, the drug may be approved for general use or sale in the United States. In some cases, a drug may require a Phase 4 trial to ensure it is safe long-term.
Overall, the FDA looks at data from clinical trials to make sure that new drugs are not too risky, work for the people they’re meant to help, and can be made safely and reliably.
What types of investigational HIV drugs are being studied?
HIV treatment has come a long way since the first antiretroviral drug (zidovudine) was approved in 1987. Today, there are over 40 FDA-approved drugs used to treat HIV. Most people with HIV can live a normal lifespan by taking a combination of two or more HIV drugs.
Although modern HIV treatment regimens are generally safe and effective, they still have some limits, and researchers are looking for ways to make them even better. The table below outlines examples of common limitations and solutions under investigation.
Drug Limitation | Problem Rationale | Investigational Solution | Expected Benefit |
|---|---|---|---|
| Daily Dosing | Taking medicine every day can be hard to maintain long term | Long-acting therapies (monthly injections, implants) | Fewer missed doses and easier medication adherence |
| Side Effects | Nausea, weight changes, and other side effects can make it hard to take medicines | New drug formulations with fewer side effects | Helps people stay on treatment without missing doses due to discomfort |
| Drug Interactions | Some HIV medicines interfere with other prescriptions, foods, or supplements | Improved drug design to reduce the likelihood of drug interactions | Safer and easier to take alongside other medications, which can also add treatment options |
| Long-Term Health Effects | Some HIV medicines may impact health over time, with issues such as reduced kidney function, bone weakness, or reduced heart health | Safer alternatives for long-term use | Minimizes organ damage or other health problems over time |
| Drug Resistance | Some forms of HIV don’t respond to certain treatments | New options for drug-resistant HIV | Provides more effective treatments for people with drug-resistant HIV |
| Special Health Needs | Pregnancy and other conditions may limit certain treatment choices | Targeted therapies designed for specific groups | Ensures safe and effective options for all people with HIV |
| HIV Not Fully Eliminated | HIV stays in the body even with treatment, known as a latent HIV reservoir | Cure-focused research targeting hidden virus reservoirs | Aims to cure or remove HIV from the body, rather than solely achieving an undetectable viral load |
| Limited Prevention Methods | Few drugs are approved by the FDA for HIV prevention | New preventive treatments, including long-acting pre-exposure prophylaxis (PrEP) | Expands options to reduce chances of HIV transmission and makes it easier to take PrEP |
| Challenges in Prevention Access | No HIV vaccine exists, and PrEP may be costly or difficult to take if daily medicines are prescribed | Vaccine research + new PrEP formulations | Makes prevention simpler and more widely available |
In addition to traditional HIV medicines, HIV researchers are continuously studying investigational vaccines to prevent and treat HIV. The goal of a preventive HIV vaccine is to prevent HIV in people who do not have HIV but may be exposed to the virus.
A safe and effective HIV treatment vaccine (also called a therapeutic HIV vaccine) could prevent HIV from advancing to acquired immunodeficiency syndrome (AIDS), replace the daily use of HIV medicines, and help prevent HIV transmission.
To learn more about HIV vaccines, read the HIVinfo What is a Preventive HIV Vaccine? and What is a Therapeutic HIV Vaccine? fact sheets.
How are clinical trials of investigational drugs conducted?
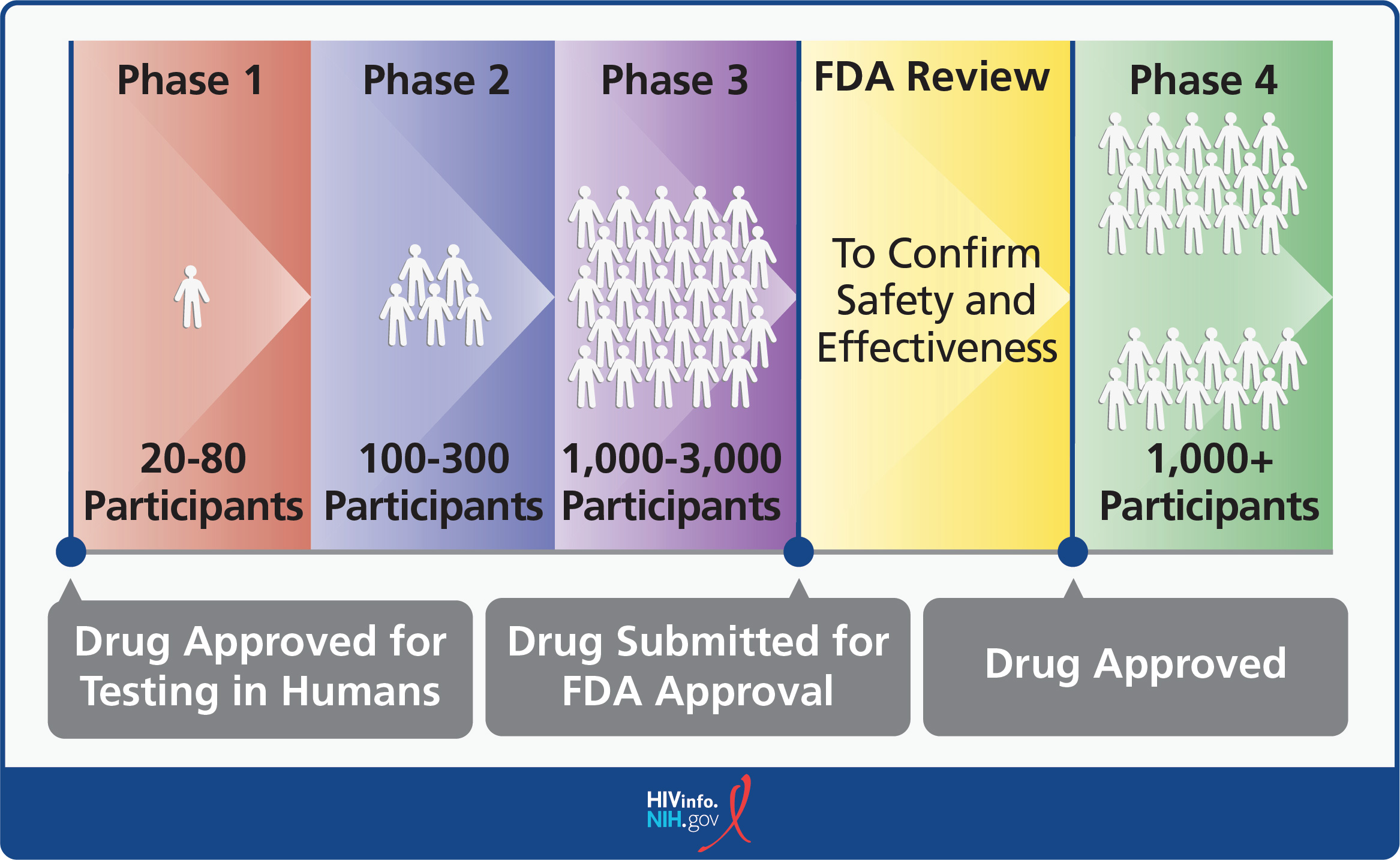
Clinical trials are conducted in phases. Each phase has a different purpose and helps researchers answer different questions about the investigational drug.
- Phase 1 trial: Initial testing in a small group of people (20-80) to make sure the drug does not pose unacceptable risks. At this point, researchers also look for signs that the new drug or treatment is effective.
- Phase 2 trial: Testing in a larger group of people (100-300) to confirm the drug works as intended and to further evaluate its safety.
- Phase 3 trial: Continued testing in large groups of people (1,000-3,000) to confirm the drug’s effectiveness, monitor side effects, compare it with standard or equivalent treatments (to see if it is better than current medicines), and collect information to ensure that the investigational drug can be used safely.
In most cases, an investigational drug must be proven effective and must show continued safety in a Phase 3 clinical trial to be considered for approval by the FDA for sale in the United States.
In rare cases, drugs go through the FDA’s Accelerated Approval Program and are approved before a Phase 3 clinical trial is complete. Accelerated approval requires early data suggesting benefit for serious conditions (such as some cancers) that lack effective treatments. Additional studies are still required to prove the drug is safe and effective. If the drug is not proven to be safe and effective, it may be withdrawn.
- Phase 4 trial: Ongoing tracking that occurs after a drug is approved by the FDA for sale in the United States. The purpose of the tracking is to seek more information about the drug’s risks, benefits, and optimal use. Phase 4 trials are not always required.
For more information, read the HIVinfo HIV and AIDS Clinical Trials fact sheet.
How can a person find a clinical trial that is studying an investigational HIV drug?
There are several ways to find an HIV and AIDS clinical trial that is searching for volunteer participants:
- To find an HIV and AIDS clinical trial that is studying an investigational HIV drug, use the “Find Studies” search feature on ClinicalTrials.gov.
- For help with your search, call a Clinicalinfo health information specialist at 1-800-448-0440 or email [email protected].
- Join ResearchMatch, which is a free, secure online tool that makes it easier for the public to become involved in clinical trials.
Not everyone will be able to participate in clinical trials using investigational HIV drugs. Many studies have strict criteria on who can participate in the study, including factors like age, sex, health, other conditions or infections, and sexual orientation.
Some people may not be able to join a clinical trial because the study requires commitment to many appointments or is only available in a specific area.
Are investigational HIV drugs available for use outside of a clinical trial?
In some cases, an investigational HIV drug may be available through an expanded access program (sometimes called “compassionate use program”). Expanded access programs allow for the use of an investigational drug outside of a clinical trial to treat a person who has a serious or immediately life-threatening disease and who has no FDA-approved treatment options.
Drug companies must have permission from the FDA to make an investigational drug available for expanded access. People seeking expanded access to an investigational HIV drug should talk to their health care provider to see if they qualify to participate in an expanded access program.
Is it safe to use an investigational HIV drug?
One goal of HIV research is to identify safer HIV medicines that are equally or more effective than existing HIV medicines. Researchers try to make clinical trials as safe as possible. However, taking an investigational HIV drug can involve both benefits and risks.
The most notable risks of taking investigational drugs include unexpected side effects from the drug, which may be unpleasant, serious, or even life-threatening.
The exact benefits and possible risks of participating in a clinical trial or an expanded access program are explained before someone starts using an investigational drug through a process called informed consent.
How can a person find more information on investigational HIV drugs?
To find more information on an investigational HIV drug, use the Clinicalinfo Drug Database, which includes up-to-date information on many investigational HIV drugs.
This fact sheet is based on information from the following sources:
From the National Institutes of Health (NIH):
- NIH Clinical Research Trials and You: The Basics
- NIH Clinical Research Trials and You: Finding a Clinical Trial
From the National Institute of Allergy and Infectious Diseases (NIAID):
From the U.S. Food and Drug Administration (FDA):
Also see the HIV Source collection of HIV links and resources.
